The big picture: Researchers at Purdue University are working on a way to increase a battery's lifespan while simultaneously making it more stable and reducing charging time. It's a promise we've heard time and again from the scientific community, but could they actually be on to something plausible this time?
As Purdue University's Kayla Wiles highlights, the researchers created a net-like structure called a "nanochain" out of antimony, a chemical element with the symbol Sb and atomic number 51 that is used to enhance the charge capacity of lithium ions in batteries.
In testing with coin cell batteries, the team observed that after a 30-minute charge, units with nanochain electrodes achieved double the lithium ion capacity compared to batteries with graphite electrodes which is most commonly used in today's batteries. This was true over 100 charge / discharge cycles.
Some commercial batteries already use composites similar to antimony as an electrode but the material tends to swell as it takes in lithium ions, sometimes by as much as three times. This, as you could imagine, leads to serious safety hazards.
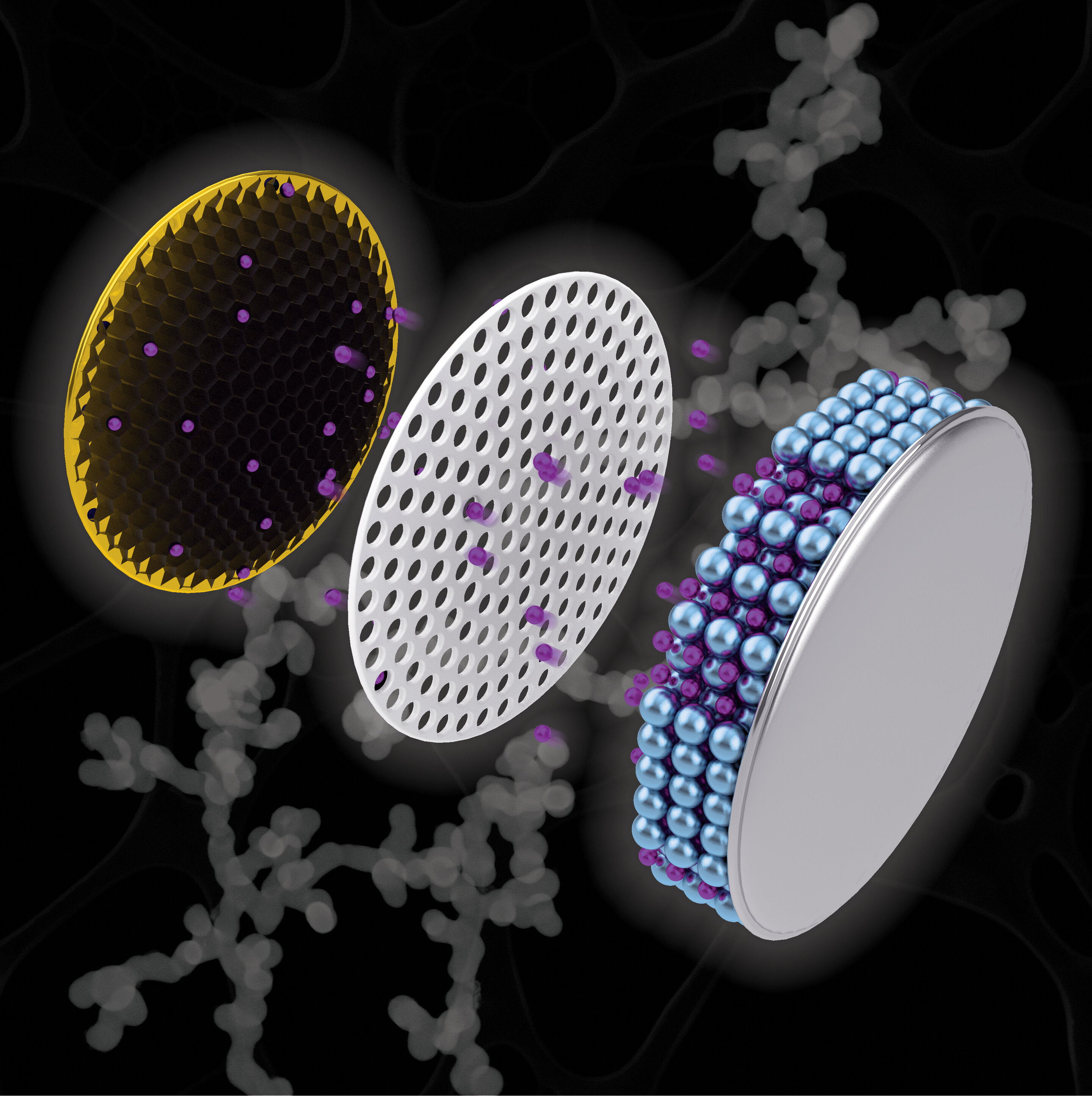
To combat the expansion, Purdue scientists applied a reducing agent and a nucleating agent to accommodate the required expansion. The reducing agent, ammonia-borane, creates empty spaces inside the nanochain, affording room for expansion and helping to suppress electrode failure.
Vilas Pol, a Purdue associate professor of chemical engineering, said that in testing, "there's essentially no change from cycle one to cycle 100, so we have no reason to think that cycle 102 won't be the same."
Of course, theory and actuality are two very different things. The truth of the matter is, a lot more testing is needed to determine the long-term safety of nanochain electrodes. It's a great first step that it survived 100 charge / discharge cycles but in the real world, batteries go through thousands of these cycles over their lifetime.
