Recycling is certainly a useful process, and likely a necessary one if modern society is ever going to achieve near-complete sustainability. However, it is not perfect - as noted by Ensivo, recycling isn't perfectly efficient for all types of materials. Certain metals, such as aluminum, can be easily be melted down and re-used with very few lost materials.
Plastics, on the other hand, are an entirely different story. As the site points out, most plastics tend to retain their previous forms in some way when recycled, meaning you couldn't easily melt down a plastic bottle and turn it into fiberglass. This is because plastics are made up of monomers, which when combined together create polymers. Apparently, it's remarkably difficult to extract the raw monomers individually; they tend to stick together.
Researchers from Lawrence Berkeley National Laboratory seem to have found a solution to this problem. As Ensivo reports, the scientists in question are using "triketones" and "amines" to construct a different type of polymer; one that responds much better to being broken down into its base components.
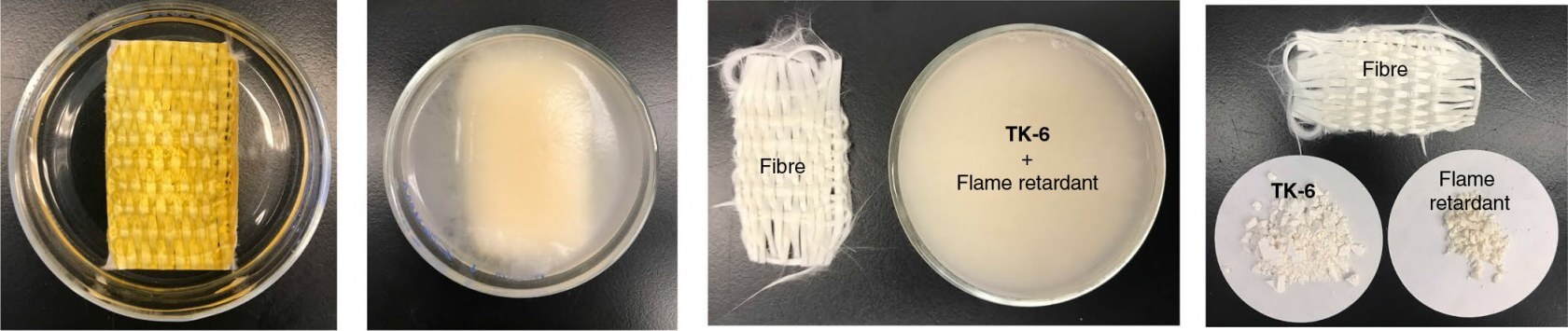
By using acid and other chemical processes, researchers were able to break down flame-resistant fiberglass weave into TK-6 powder, a piece of fiber, and a flame-retardant "additive." While it's unclear whether or not this process is completely lossless, it seems to come pretty close, and it's certainly a major achievement for the recycling industry regardless.
Now, the materials obtained from this extraction process can be used to create any new type of plastic researchers desire.
To be clear, this process will only be truly beneficial moving forward. Plastic makers would need to start using the triketones and amines mentioned before on a large scale to enable this form of recycling in the future. However, this is the first necessary step in the process, and it's nice to see such an old and seemingly static industry receive real innovation.
